Who Needs the Treatment ?
Based on the signs, symptoms and the type of SMA, the Health Care Professional decides the treatment plan.
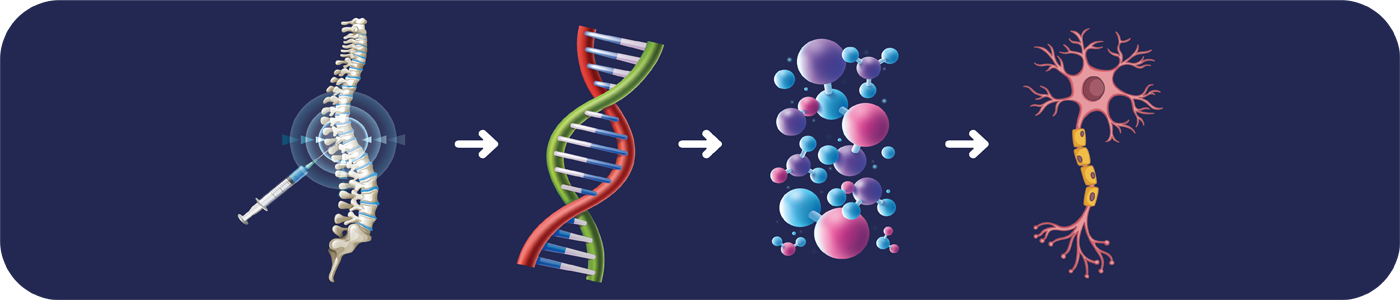
Following the identification of the underlying genetic aetiology of SMA, therapy strategies with drug like Spincura that target the SMA-related genes can be opted. Early treatment can significantly extend life expectancy and enhance the quality of life for individuals.
Signs and Symptoms of
SMA.
Based on the signs, symptoms and the
type of SMA, the Health Care Professional decides the treatment duration and effective working of
SpinCura.
Muscle weakness
Unable to Stand and Walk
Involuntary trembling (tremors) in their
fingers
Scoliosis(spine that curves side-to-side)
Respiratory muscle weakness that can be
life-threatening
SMA Type:
SpinCura (Nusinersen) is specifically used to treat
various types of SMA as specified below:
Assist untreated children who
does
not have the muscular strength to sit unassisted, with a life expectancy of around 2 years
in SMA type 1 and SMA type 2 patients.
It can fight against the
respiratory challenges and control the progression of the disease in patients with SMA of
all types.
It helps maintain their daily
routine by decreasing the muscle weakness in the patients with type 3 and type 4 SMA that
emerges in children of 18 months old or older.
In situations of aggressive
SMA of
types 1 and 2, SpinCura treatment prolongs the life span of the patients.
Safety Profile:
Blood and urine testing:
Individuals on
SpinCura may be at similar risk because comparable drugs have been shown to raise the risk of
bleeding and kidney injury.
To check for indications of these
hazards, it is advised that your healthcare provider (HCP) complete blood and urine tests before
starting therapy and again before each dosage.
Common Side Effects:
Based on clinical trials, lower respiratory infections, fever,
constipation, headaches, vomiting, back discomfort, and post-lumbar puncture syndrome (a
headache caused by the intrathecal administration) were among the most frequent side
effects.
Important Safety Information
Similar medications have been associated with an increased risk of
bleeding
problems. Before you begin SpinCura medication and before each dosage, your healthcare
practitioner should run blood tests to check for indications of these risks. In the event of
sudden bleeding, get medical help.
Similar medications have been associated with an increased risk of kidney
injury, including acute renal inflammation that can be deadly. Urine testing should be done
by
your healthcare practitioner both before you begin SpinCura medication and before each
dosage in
order to check for indications of this risk.
Lower respiratory infections, fever, constipation, headaches, vomiting,
back
discomfort, and post-lumbar puncture syndrome are among the most typical adverse effects of
SpinCura.
These aren't the only potential adverse effects of SpinCura. If you would
want
medical advice regarding side effects, contact your physician. Adverse events can also be reported by calling
+91 4023544875/
76/
77
or mailing to
[email protected], you can report
adverse effects.
Inform your healthcare practitioner if you are pregnant or want to become
pregnant
before starting SpinCura.